
Whereas only one sigma bonding MO is possible, the pi. To form a triple bond, we need to have one sigma bonding MO and two pi bonding MOs.
You can find sp2 bonding when carbon has a ONE. A sigma bond () is formed by the direct head-on/end-to-end overlap. For a carbon with 1 double bond and 2 single bonds, the orbitals will become 33 's' and 66.7 'p' making it 'sp2.' If there is a triple bond and a single bond, the orbitals will adjust again to become 50 's' and 50 'p.' So to summarize - You can find sp3 bonding when a carbon has 4 single bonds. If you would use that formula for propane, number of sigma bonds will be 3 + 8 - 1 = 10, as shown above. With two electrons in the sigma bonding MO, and two electrons in the pi bonding MO, and zero electrons in antibonding orbitals, we have an overall bond order of 1 / 2 (4 0) 2, i.e., a double bond. 26K views 2 years ago Topic 4/14 - Bonding SL/HL Understandings: Covalent bonds result from the overlap of atomic orbitals. It is formed when atomic orbitals overlap. Number of sigma bonds = number of atoms - 1 (which you can determine using the molecular formula ) It is formed when atomic orbitals overlap along the internuclear axis. Also, in this particular case ( for saturated aliphatic alkanes ) you can use a formula: Polyatomic molecules edit Sigma bonds are obtained by head-on overlapping of atomic orbitals.
#Sigma and pi bonds plus
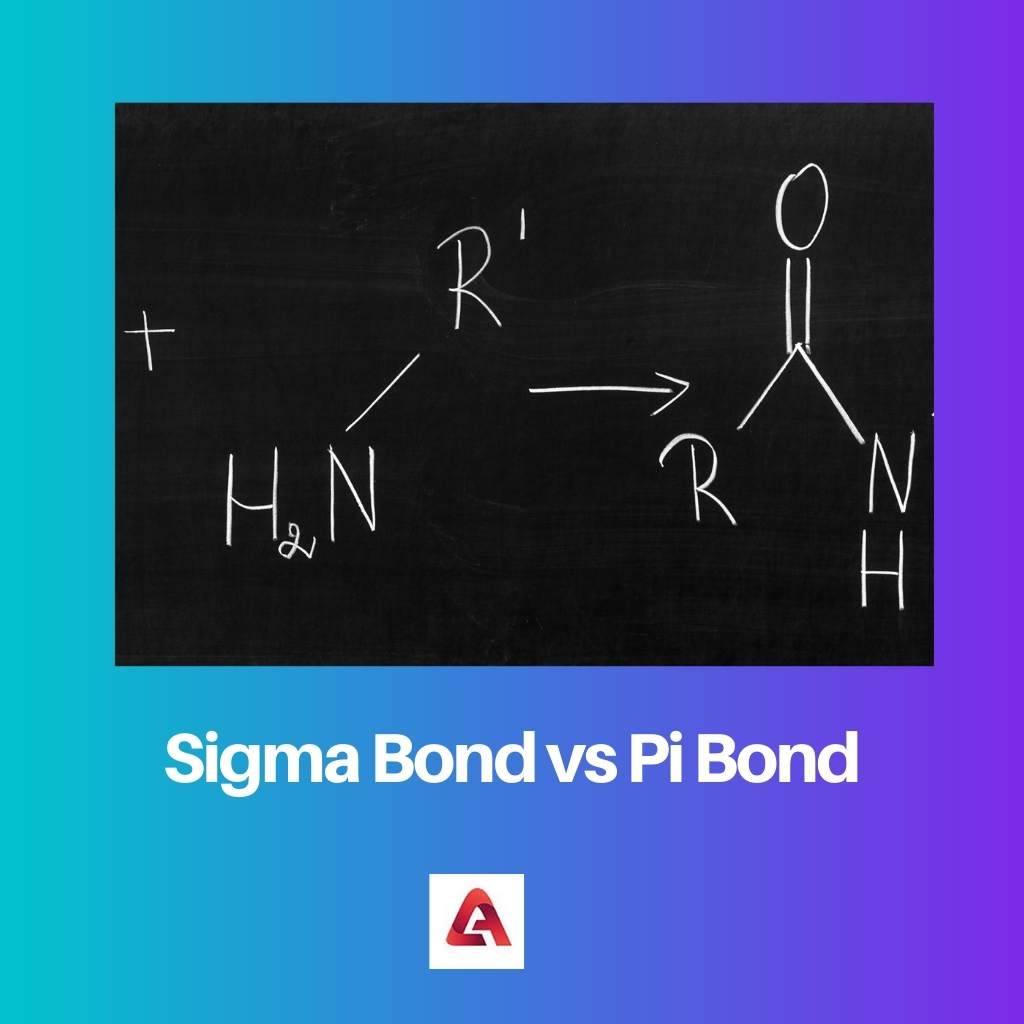
A double bond has one sigma plus one pi bond, and a triple bond has one sigma plus two pi bonds. Pi bond: Includes lateral overlapping of orbitals of two lobes of an atom to a different atom. Typically, a single bond is a sigma bond while a multiple bond is composed of one sigma bond together with pi or other bonds.

It is a saturated compound, so you will only have simple ( sigma ) covalent bonds - there are no pi bonds. Sigma and Pi Bonds A covalent bond can be classified into types: Sigma bond: Includes direct overlapping of orbitals and is one of the strongest bonds. Three sigma bonds are formed from each carbon atom for a total of six sigma bonds total in the molecule. Let's take the propane extended structure formula (which i stole from the answer above): Each simple covalent bond (represented by the line between 2 atoms) will be a sigma bond Īny other different from that ( double, triple covalent bonds ) will still have one sigma bond, the rest of the lines being counted as pi bonds
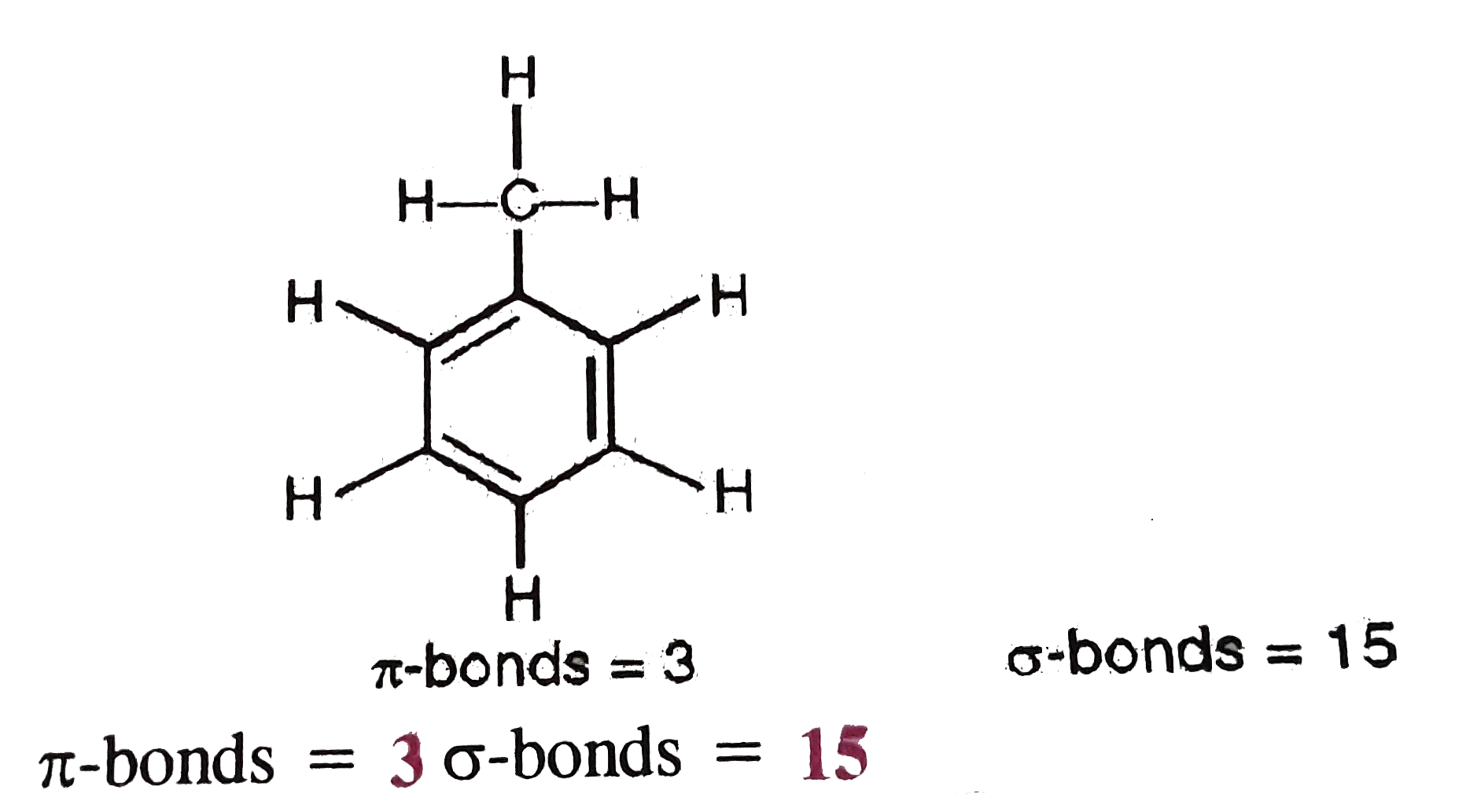
Start off by writing down the respective compund using the extended structure model. Here you have a more in-depth analysis of structure formulas. It is a saturated compound, so you will only have simple ( sigma ) covalent bonds - there are no pi bonds.


 0 kommentar(er)
0 kommentar(er)
